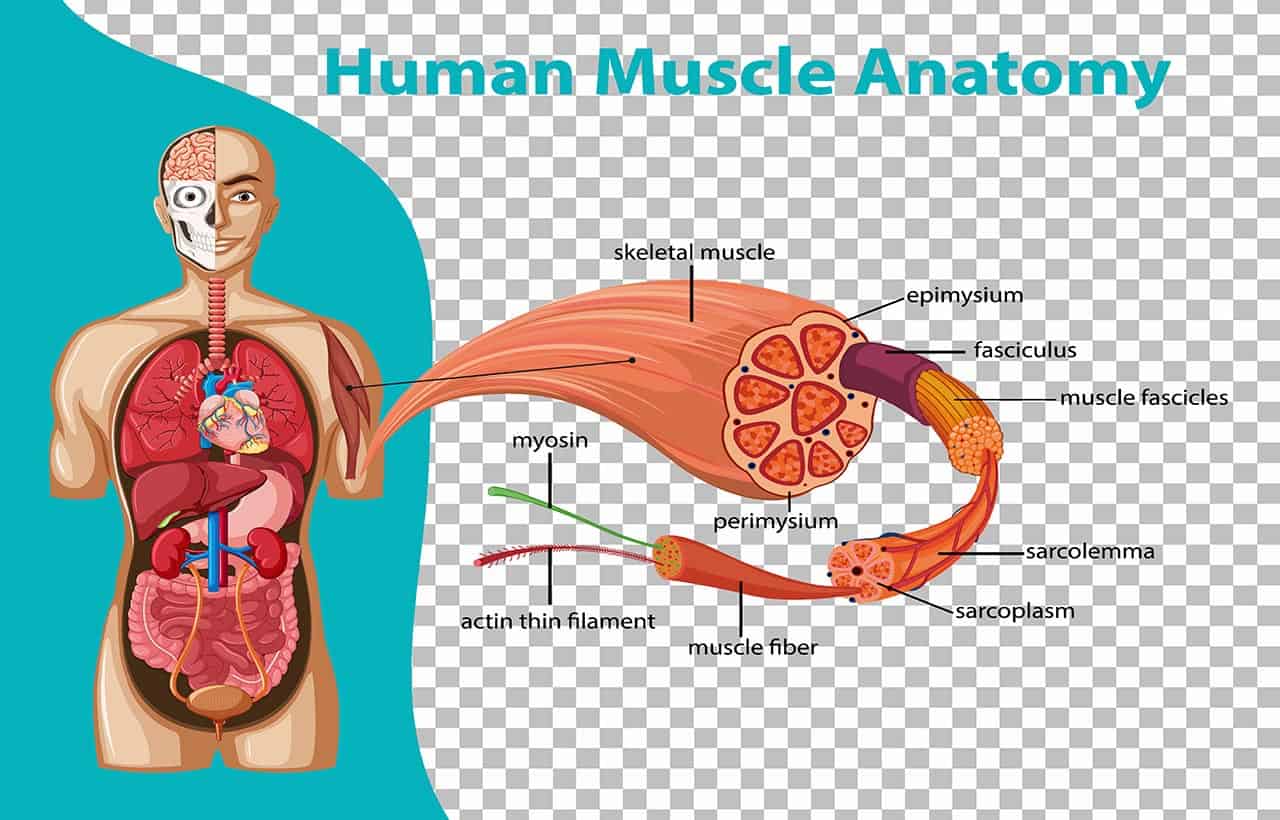
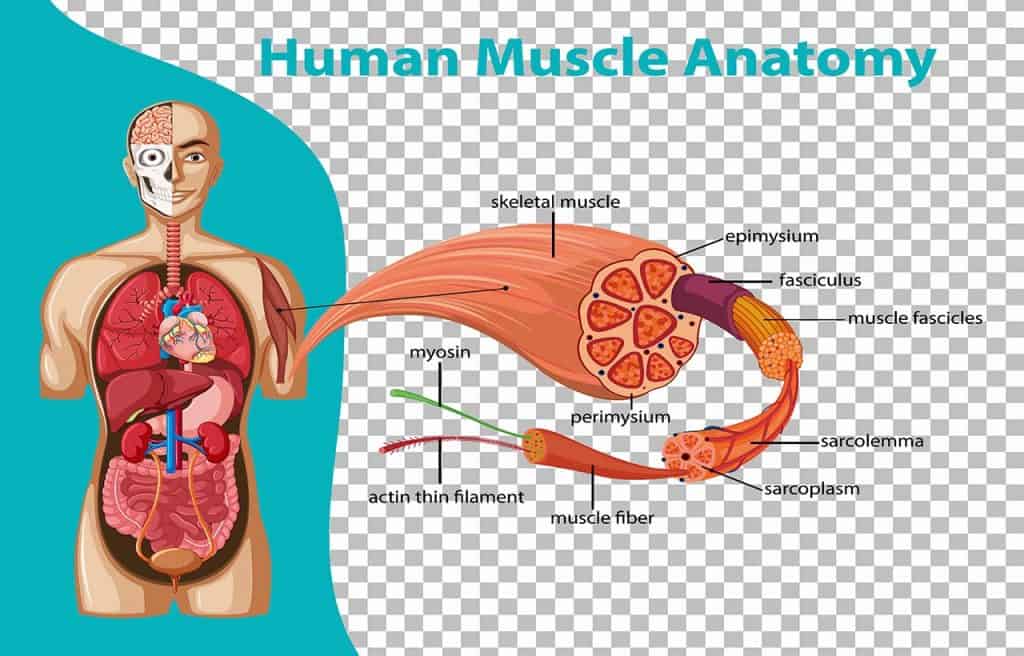
The anatomical structure of skeletal muscle is made up of muscle fibers or bundles of muscle fibers and is surrounded by and held together with connective tissue.
Although the main role of muscles is to move the bones of the skeleton, they also cause the heart to beat and form the walls of other important hollow organs.
Skeletal muscle accounts for 40-50% of total body weight. According to medical terms, it has three main functions:
- Force generation for movement
- Postural support
- Heat production during periods of cold stress
Skeletal muscle
Skeletal muscles are also known as voluntary muscles. Skeletal muscles are attached to bones by tendons and interact with every movement of every part of the body. Skeletal muscle is a voluntary muscle that is not the same as cardiac and smooth muscle.
However, like the heart muscle, skeletal muscle also has striated muscles. Thin, long, and multinucleated fibers crossed with a regular pattern of white and red stripes, giving the muscles a unique shape. Skeletal muscle fibers are held together by connective tissue and communicate with nerves and blood vessels.
The anatomical structure of skeletal muscle
Characteristics of skeletal muscle
Skeletal muscles are striated and willful or voluntary muscles. The word striated means “striped”. Skeletal muscle is the only type of muscle that the nervous system can consciously control. This is the main reason to become a voluntary muscle.
If you see skeletal muscle cells in a microscope then you will realize that this muscle looks long and cylindrical. For this reason, skeletal muscle is also called skeletal muscle fibers. Skeletal muscle fibers can be up to 1 foot long!
These long, highly specialized cells are the result of many cells fusing together, and individual nuclei are preserved when the cells fuse. As a result, skeletal muscle fibers become multinucleated. Meaning that the skeletal muscles have more than one nucleus on them.
Each nucleus regulates the metabolic needs of the sarcoplasm around it. Because skeletal muscle cells require a lot of energy, they contain many mitochondria to produce enough ATP.
The anatomical structure of skeletal muscle
Now we will learn about the anatomical structure of skeletal muscle. Skeletal muscle cells (fibers), like other cells in the body, are soft and fragile. Skeletal muscle consists of fascicles or bundles of muscle fibers and is surrounded by connective tissue. An individual skeletal muscle can be composed of hundreds or thousands of muscle fibers interconnected by a connective tissue sheath. This connective tissue forms three layers:
- Epimysium
- Perimysium
- Endomysium
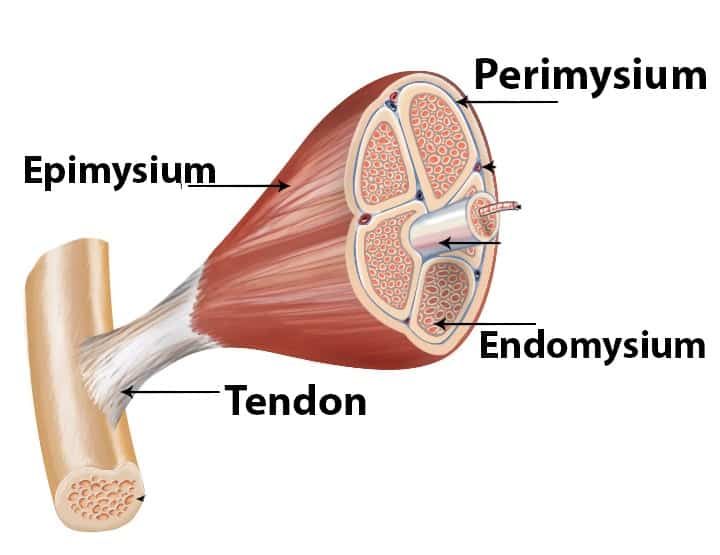
The connective tissue layer holds muscles together, connects muscles with other structures in the body, and forms tendons to connect muscles with bones. When a muscle contracts, tension is transmitted through the connective tissue, which pulls on the muscle attachment point and produces movement. Muscle fibers are long cylindrical cells that are parallel to each other.
The plasma membrane (sarcolemma) of muscle cells contains many myofibrils distributed throughout the body, among which are the contractile components of muscles. Myofibrils are composed of filaments arranged in compartments called sarcomeres.
These filaments are thick or thin and overlap with different amounts of IU depending on whether the muscle is relaxed, contracted, or stretched. This causes the appearance of stripes (alternating dark and light bands) in skeletal muscles at the microscopic level.
| This Article The anatomical structure of skeletal muscle |
Each sarcomere is located between the Z lines. The A-band is mainly composed of myosin, and its length does not change with contraction. Band I is mainly composed of actin, but there is also myosin overlap. The length of the belt changes as it shrinks.
Now let’s learn about the three layers of the muscles individually as you are trying to know to learn about the anatomical structure of skeletal muscle briefly.
Epimysium: Epimysium is the fibrous tissue that surrounds the skeletal muscle. It is a layer of dense, irregularly shaped connective tissue that surrounds the entire muscle and protects it from friction with other muscles and bones. It continues with the fascia and other connective tissue sheaths of the muscle, including the fascia. Tendons also often become thicker and more collagenous. While the epimysium in the muscles is irregular but regular in the tendons.
Perimysium: Perimysium is a connective tissue sheath that binds muscle fibers into bundles (10–100 or more) or fascia. Muscle physiology studies indicate that the fascia plays a role in the transmission of lateral contractile movements. This hypothesis was strongly supported by Emilie Passeriere, who testified to an ineffective “proximal attachment plate” in the radial wrist flexor. However, all integrative networks of the peripheral collagen network, their continuity, and inequality in muscles have not been fully studied. It is known to contain collagen types I, III, VI, and XII.
Endomysium: Endomysium locates inside the muscle and is a thin layer of areola connective tissue that surrounds each individual muscle fiber or muscle cell. Endomysium also contains capillaries and nerves. Endophasia, or internal muscle, is a thin layer of loose connective tissue that surrounds individual muscle fibers or cells. It also contains capillaries and nerves. It is overlapped by the sarcolemma, the cell membrane of muscle fibers. Endomysium is the deepest and smallest part of the muscle connective tissue. This thin layer helps create a suitable chemical environment for the exchange of calcium, sodium, and potassium. This is important for the excitation and subsequent contraction of muscle fibers.
Anatomical unit of muscle
The anatomy of muscle cells is different from the anatomy of other cells in the body, and biologists use specific terms to refer to the different parts of these cells. The cell membrane of muscle cells is called the sarcolemma, and the cytoplasm is called sarcoplasm.
Sarcoplasm contains myoglobin, which is an oxygen storage site, and granular glycogen in the cytoplasm that provides energy. Sarcoplasm also contains many tubular protein structures which contain long cylindrical structures called myofibrils.
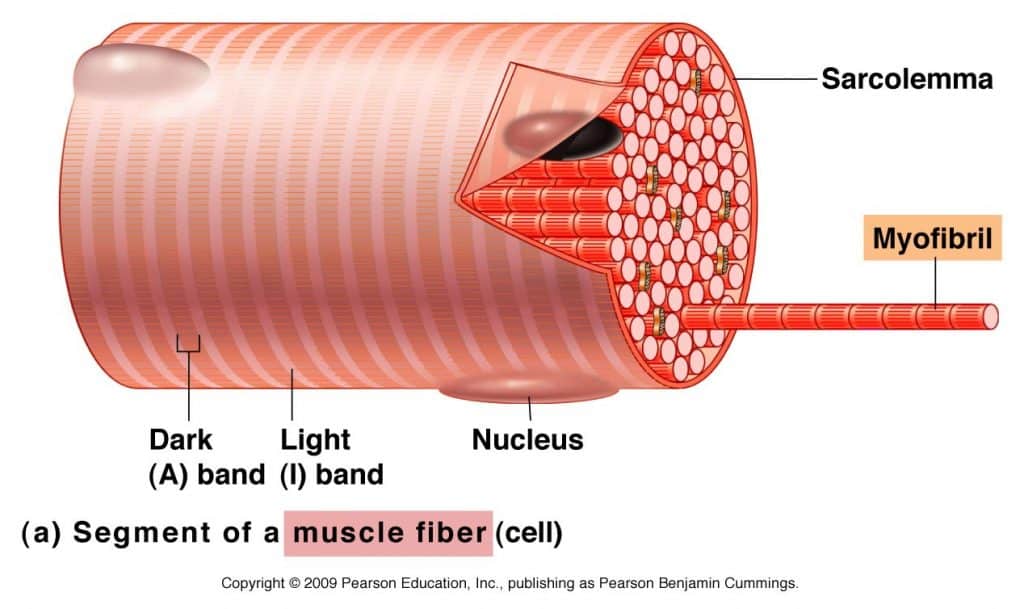
Myofibrils almost completely fill the cell and push the nucleus to the outer edge of the cell under the sarcolemma. Each of the many myofibrils has a light and dark bands located side by side. This gives the cell its striated appearance.
Light-colored bands are called I-band, and dark-colored bands are A bands. In the middle of the I-band is a line called the Z-line (or disc). At the center of the A (or dark) band is a bright area called the H zone. In the middle of the H zone is another line called the M line. The exact arrangement of these features is because of a chain of functional units in the myofibrils, sarcomeres.
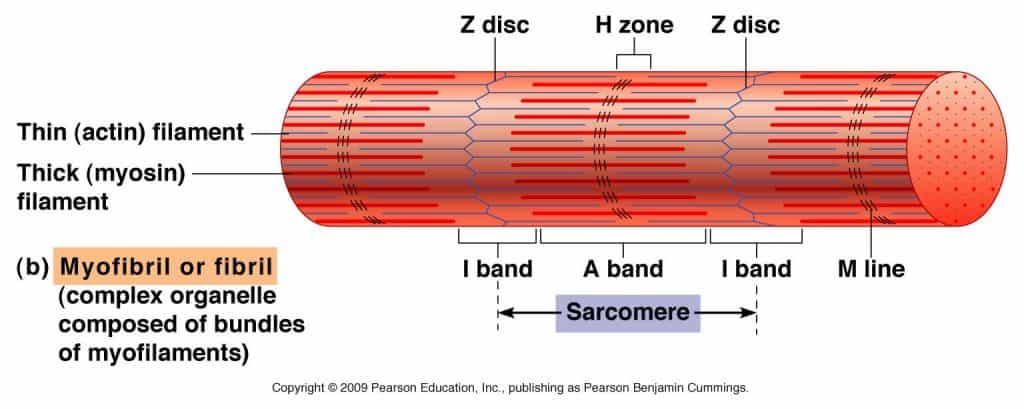
The sarcomere comprises various individual protein components. A portion of these proteins is string-like proteins called myofilaments. There are two significant sorts of myofilaments, a) Thick myofilaments and b) Thin myofilaments
Thick myofilament: It is also known as myosin filament. Thick myofilaments are comprised of proteins molecules known as myosin. The myosin molecules are formed like golf clubs with long shafts. Myosin forms bundles of thick myofilaments, where the head of the “golf club” protrudes at each end of the filament, and the shaft forms a “bare” area in the middle of the filament. The head of the thick filament forms a connection with another type of filament, the fine actin myofilaments. These attachments are called cross bridges. The head is also the part of thick filaments, which uses the energy of ATP molecules to drive muscle contraction.
Thin myofilament: It is also known as actin filament. Thin myofilaments are composed of the protein actin. The thin myofilaments have attachment sites, and the heads of the thick filaments adhere to these attachment sites.
Sarcomere: The sarcomere addresses the basal contractile unit of striated muscles. The sarcomere addresses the basal contractile unit of striated muscles. A single myofibril is made out of these short structural units orchestrated end to end, which contract because of the relative sliding of thick (myosin) over thin (actin) filaments. Structural features of the sarcomere bundles of parallel thick and thin filaments combined by two thwart structures.
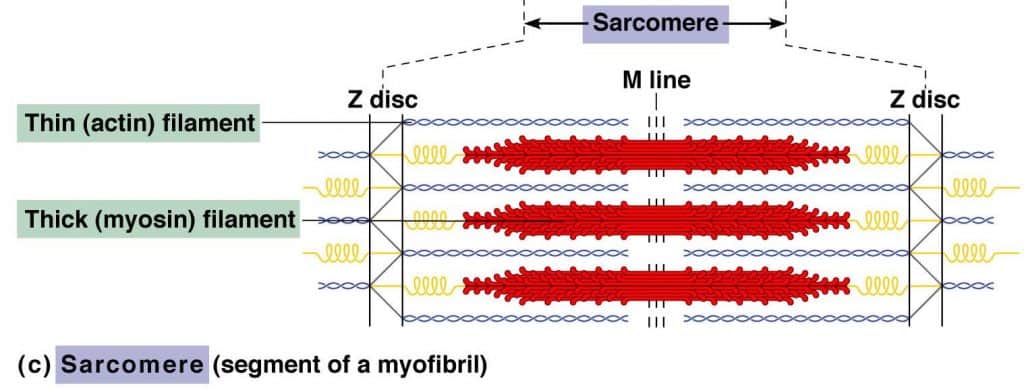
The Z lines and the M lines are attached by thwart filaments to the sarcolemma or to the adjoining myofibrils. Sarcomeres provide for skeletal and cardiovascular muscles their striated appearance with I bands encompassing the Z lines, followed by A bands. The A bands contain a paler area called the H zone and in the center the M line.
Physiology of skeletal muscle contraction
The physiology of skeletal muscle or skeletal muscle contraction is also named as sliding filament theory. So on the basis of this, sliding filament theory is a mechanism for muscle contraction at the cellular level.
Essentially, all myofibrils are made up of tiny fibers called myofibrils. It contains smaller structures called actin and myosin filaments.
These filaments slide in and out to form muscle contractions. For this reason, it is called the sliding filament theory. The smallest units of skeletal muscle are called sarcomeres and can contract.
The smallest unit of skeletal muscle is called the sarcomere that can contract. Sarcomeres repeat the same thing again and again along the length of the myofibril.
Here is the thing that occurs exhaustively. The interaction of a muscle contracting can be separated into 5 segments:
Segment 1: Nerve impulses reach the neuromuscular junction and trigger the release of the chemical called acetylcholine. The presence of acetylcholine causes depolarization of the motor endplates, which pass through the transverse tubules of the muscles, releasing calcium (Ca+) from the sarcoplasmic reticulum.
Segment 2: Within the sight of high concentrations of Ca+, the Ca+ binds with troponin, changing its shape thus moving tropomyosin from the dynamic site of the Actin. The Myosin filaments would now be able to connect to the Actin, which formed a cross-bridge.
Segment 3: The breakdown of ATP discharges energy which empowers the Myosin to pull the Actin fibers inwards thus shortening the muscle. This happens along the whole length of each myofibril in the muscle cell.
Segment 4: The Myosin withdraws from the Actin and the cross-bridge is broken when an ATP molecule ties with the Myosin head. At the point when the ATP is separated from the Myosin head can again join to an Actin binding site further along the Actin filament and rehash the ‘power stroke’. This continued pulling of the Actin over the myosin is frequently known as the ratchet mechanism.
Segment 5: This muscular contraction process can last for as long as there are sufficient ATP and Ca+ stores. When the impulse stops the Ca+ is pumped back to the Sarcoplasmic Reticulum and the Actin gets back to its resting position making the muscle lengthen and relax.
Visual view of the anatomical structure of skeletal muscle
It will be best if you see this video minimum of one time. Cause if you see this video then the whole lesion (or article) will memorize easily and it would be unforgettable for you!
Resources
- Sarparanta, J. , Blandin, G. , Charton, K. , Vihola, A. , Marchand, S. , Milic, A. , … Udd, B. (2010). Interactions with M‐band titin and calpain 3 link myospryn (CMYA5) to tibial and limb‐girdle muscular dystrophies. Journal of Biological Chemistry, 285(39), 30304–30315. [PMC free article] [PubMed] [Google Scholar]
- Sarrazin, S. , Lamanna, W. C. , & Esko, J. D. (2011). Heparan sulfate proteoglycans. Cold Spring Harbor Perspectives in Biology, 3(7), a004952. [PMC free article] [PubMed] [Google Scholar]
- Sartori, R. , Milan, G. , Patron, M. , Mammucari, C. , Blaauw, B. , Abraham, R. , & Sandri, M. (2009). Smad2 and 3 transcription factors control muscle mass in adulthood. American Journal of Physiology: Cell Physiology, 296(6), C1248–C1257. [PubMed] [Google Scholar]
- Sato, T. , Rocancourt, D. , Marques, L. , Thorsteinsdóttir, S. , & Buckingham, M. (2010). A Pax3/Dmrt2/Myf5 regulatory cascade functions at the onset of myogenesis. PLoS Genetics, 6(4), e1000897. [PMC free article] [PubMed] [Google Scholar]
- Scharner, J. , & Zammit, P. S. (2011). The muscle satellite cell at 50: The formative years. Skeletal Muscle, 1(1), 28. [PMC free article] [PubMed] [Google Scholar]
- Schiaffino, S. , & Mammucari, C. (2011). Regulation of skeletal muscle growth by the IGF1‐Akt/PKB pathway: Insights from genetic models. Skeletal Muscle, 1, 4 10.1186/2044-5040-1-4 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Schiaffino, S. , & Reggiani, C. (2011). Fiber types in mammalian skeletal muscles. Physiological Reviews, 91(4), 1447–1531. [PubMed] [Google Scholar]
- Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999 Sep;126(18):4053-63. [PubMed]
- Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development. 1997 Dec;124(23):4729-38. [PubMed]
- Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf). 2011 Jul;202(3):271-84. [PMC free article] [PubMed]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand. 2000 Apr;168(4):511-8. [PubMed]
- Dodd LR, Johnson PC. Diameter changes in arteriolar networks of contracting skeletal muscle. Am J Physiol. 1991 Mar;260(3 Pt 2):H662-70. [PubMed]
- Heuser JE, Salpeter SR. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150-73. [PMC free article] [PubMed]
- Slater CR. The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions. Int J Mol Sci. 2017 Oct 19;18(10) [PMC free article] [PubMed]
- Caire MJ, Reddy V, Varacallo M. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 24, 2020. Physiology, Synapse. [PubMed]
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010 Apr;137(7):1017-33. [PMC free article] [PubMed]
- Ottenheijm CA, Granzier H. Lifting the nebula: novel insights into skeletal muscle contractility. Physiology (Bethesda). 2010 Oct;25(5):304-10. [PubMed]
| Read More Great Articles What bones make up the shoulder do you know? |